
 |
 |
|||
| An introduction to molecular parasitology and trypanosomes
|
| The DNA double helix | What is Molecular Parasitology? | |
 |
Parasitology is the study of parasites: organisms that can only survive by living on others. This is a very broad definition! Among micro-organisms, parasites include viruses, bacteria and protozoa. Virology and bacteriology are separately defined disciplines, so the term parasitology is generally applied to nucleated unicellular and multicellular organisms. Among these are the single-celled protozoa that cause malaria, leishmaniasis, trypanosomiasis, toxoplasmosis, and the multicellular worms, which range from microscopic to the easily visible! Parasitic diseases remain a major problem in many parts of the world. They thrive in poor social, political and economic conditions, and for the absence of vaccines and a severe shortage of affordable effective drugs. Molecular Parasitology is the study of parasites and their interactions with their hosts, at the molecular level. As in all of modern biology, we increasingly strive to understand the molecular mechanisms that enable organisms to survive, proliferate, and interfere with the health of their hosts. The foundation of molecular biology is the recognition that the information required to specify cellular process is encoded in DNA, the 'self-replicating' double helix (left), which is genetic material. Genes encode the amino-acid sequence of proteins and proteins form the machinery that engineers the characteristics of individual cells and organisms. Using proteins, cells create carbohydrates, lipids and other complex molecules. Through understanding parasitism at the molecular level, we hope to devise effective remedies for parasitic diseases. |
|
| What are Trypanosomes? | ||
|
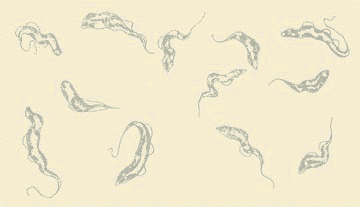
Trypanosomes have been around for more than 300 million years. They are microscopic unicellular protozoa that are ubiqitous parasites of insects, plants, birds, bats, fish, amphibians and mammals. Because they have been around for so long, they and their natural hosts have evolved together to ensure their mutual survival. Trypanosomes can be found from Nairobi to New York, from Sydney to San Francisco, and from Birmingham to Buenos Aires. Fortunately, few species of trypanosomes are pathogenic. Trypanosomes, and other parasites, mainly cause disease when they spread to new hosts, like humans and their domestic animals, especially recent imports into endemic areas of species that diverged since continents separated. We generally associate trypanosomes with diseases in Africa and South America. The related Leishmania organisms have a wider distribution. African trypanosomiasis is commonly known as Sleeping Sickness in humans and Nagana (meaning 'loss of spirit' in the Zulu language) in cattle. Click here to learn more about why African trypanosomiasis is such a serious disease. Trypanosoma cruzi causes Chagas' Disease, a chronic human infection prevalent throughout Latin America, extending within the southern borders of the USA. The scanning electron micrograph (right) of a single trypanosome shows how the flagellum, which is responsible for movement, emerges from the posterior end (left in this picture), then adheres to the body of the cell, causing the characteristic appearance of the moving cells. In contrast to what one normally expects, the direction of motion is towards the end of the flagellum. Another page links to extensive information on the avoidance and treatment of African Sleeping Sickness and current efforts to develop effective and safe drug therapy. |
 |
| This short movie illustrates the trypanosome's characteristic body movement in a sample of infected blood, among the red blood cells. The diameter of a blood cell is about seven micrometers (seven millionths of a meter): the length of these trypanosomes is about 20 micrometers. | ||

Click on the photo to watch a short © video of a Tsetse feeding. Posted by permission of Sung-Woong Park, Producer of a 4-part documentary series titled 'Parasite' created by the EBS (Educational Broadcasting System) of South Korea and first broadcast in July 2013. This segment was recorded at the Liverpool School of Tropical Medicine tsetse facility. |
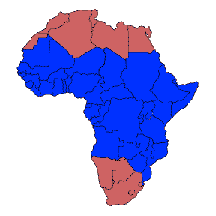
How are Trypanosomes Transmitted? Transmission of human-infective trypanosomes occurs primarily through insects that feed upon us. The insect vectors, however, are very specific. The reason is that transmission usually requires the parasite to multiply and undergo specific developmental transitions in the insect (see Life Cycles). In fact, trypanosomes may originally have been purely parasites of insects. Adaptation to mammalian hosts was probably a late event in their evolution.To learn more about the history of African Trypanosomiasis, acquire a copy of this very readable 2006 review by Ian Maudlin: African trypanosomiasis. Annals of Tropical Medicine and Parasitology 100:679-701. The so-called African trypanosomes, like the well-studied Trypanosoma brucei, are largely restricted to equatorial Africa (right), because they are primarily transmitted by specific flies commonly known as the Tsetse (left), which are only found in equatorial Africa. Some species of African trypanosomes can be transmitted by biting flies (especially Trypanosoma evansi in camels) or sexually (Trypanosoma equiperdum, in horses). Trypanosoma evansi and Trypanosoma vivax are also found in Brazil, where they infect cattle and horses and are distributed by biting flies and vampire bats. The photograph (above left) of a Tsetse (Glossina morsitans) feeding on a human volunteer was provided by Dr Leo Jenni, Swiss Tropical Institute, Basel. |
 |
| Transmission via infected blood is a particular concern for Chagas' Disease, caused by Trypanosoma cruzi, and is the reason why one of the questions for blood donors in the USA is 'Have you ever had Chagas' Disease, or lived in an area where it is endemic?' Otherwise, the specific vectors for transmission of Trypanosoma cruzi are crawling bugs like the one shown below left, which infest houses and the surrounding flora in endemic areas. In the 1980s, Brazil honored discoverers of the disease and its cause, Carlos Chagas and Oswaldo Cruz (below), on two banknotes, unfortunately in a rapidly devalued currency. | ||
| Triatoma infestans | Carlos Chagas | Oswaldo Cruz |
 |
 |
 |
| Why do We Study Trypanosomes? Trypanosomes remain a constant threat to the lives of humans, cattle, and other domesticated animals, throughout large regions of Africa and South America. Trypanosome infections are invariably fatal, taking weeks or years depending upon the species and the virulences of individual strains. Our studies of these organisms focus on why our immune systems fail to eliminate them. There are no vaccines for these diseases. We are trying to understand and overcome the obstacles that trypanosomes present to vaccine development. There are no modern drugs to treat diseases caused by trypanosomes. The few drugs that are available to treat African trypanosomiasis have their origins in the first half of the 20th century and are all very toxic. The limiting factor for drug development is the lack of resources and motivation for making new drugs for exotic diseases in distant countries that lack the ability to pay for them. Further information on the disease can be found at the World Health Organization Division of Control of Tropical Diseases. Information about WHO Research and Traiing Programs is also available. | ||
| Trypanosomes have also turned out to have great intrinsic scientific interest. Trypanosomes have evolved very differently from the better studied organisms, especially bacteria, yeast and animal cells, on which the foundations of biology have been built. Some of the most basic cellular and genetic pathways have developed very differently in trypanosomes. Some important processes that were discovered in trypanosomes are now known to be important in mammalian cells. Studies of some processes that operate in a more primitive (differently evolved, to be politically and probably scientifically correct) fashion in trypanosomes can help us to understand how the very complex mechanisms of animal cells have evolved. For example, the chromosomes of trypanosomes and humans share many properties, but also differ in significant ways. Coincidentally, the chromosome ends (telomeres) of humans and trypanosomes share certain fundamental features and purposes, but trypanosomes appear to exploit these features in other important ways. Energy generation and other metabolic pathways differ in trypanosomes: sometimes they are simpler but in other ways their regulation is rather complex. Some of these differences can be exploited to generate more effective and safer drugs for trypanosomiasis. | ||
| Acknowledgements I would like to thank Stephen Burley, Ketan Gajiwala, Leo Jenni, John Kuriyan, Tony Popowicz and Hynek Wychterle, for generously providing images and other assistance. | ||